Specialists at the Eurasian Academy of Good Practices' GxP audit training center consistently identify recurring, systemic errors made by manufacturers during inspections.
A common issue is that many companies still approach external audits or pharmaceutical inspections as isolated events, rather...
Inspections of pharmaceutical production sites for compliance with GMP standards are an integral part of the drug quality assurance system. The Pharmaceutical Inspectorate of the Russian Ministry of Industry and Trade discussed the specifics of an inspector’s work, along...
Yekaterina Repkina, Director of the Personnel Training Department of the HR Directorate of R-Pharm
Modern pharmaceutical production involves many complex operations and meticulous quality control, so the requirements for staff qualifications are very high. The development of production sites must...
The development of Russia's pharmaceutical industry requires significant and systemic investment. The state offers businesses a range of support instruments—from tax benefits to guaranteed sale mechanisms. Each has its own features, limitations, and strategic rationale. The GxP News editorial...
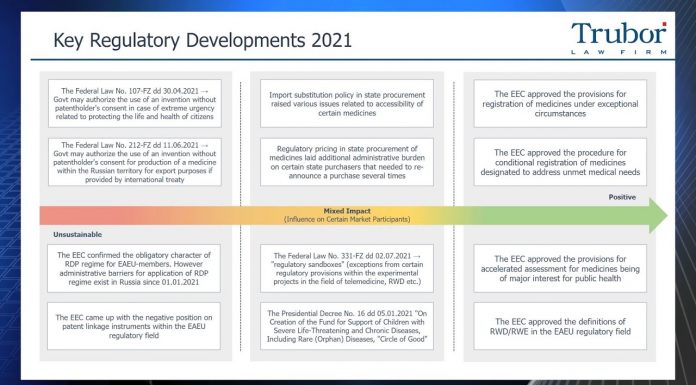
This video update demonstrates the key regulatory developments in the Russian pharmaceutical sector in 2021, the forecasts for 2022 and the open statistic data on the KPIs of national projects "Healthcare" and "Demography".
At the level of the Eurasian Economic...
The new video briefly looks at the key regulatory developments of 2020 in the sphere of IP protection, pricing and state procurement, EAEU common market and other issues.
IP protection issues
This year the topic of compulsory licensing remained under consideration....
A new video is related to the legal procedures for import, funding, and usage of unregistered medicines in Russia and provides a brief overview of the applicable regulations, court practice and ethical guidelines.
Pharmaceutical products and therapies which do not...
“The highlight is really the acceleration of access to our innovation. We always have provided and registered our products here, but this year was truly a leap year. We registered for example four products two in HIV one antibiotic...